| People | Research |
|
Label-free characterization
of temperature-dependent biomolecular binding We are also exploring MEMS-based characterization of temperature dependent biomolecular binding by matrix assisted laser desorption/ionization mass spectrometry (MALDI-MS). This method has the distinct advantage of being label-free in that it does not use fluorescent or radioactive labeling groups, which could interfere with bimolecular binding events. Our temperature-dependent measurements make use of a microfluidic platform we have developed for aptamer-based affinity purification and enrichment. At each of a series of temperatures, an analyte sample with a non-binding analyte (a reference standard) is introduced to the receptor-functionalized solid surfaces and incubated for a sufficient time period, so that some of the analyte molecules bind with the receptor, while the reference standard remains in solution. The sample, now containing non-binding analyte molecules and the standard, is transferred for MALDI-MS analysis. The normalized mass spectral peak of the analyte, defined as the ratio of the mass spectral peaks of the analyte and reference standard, will vary at the different temperatures. This relationship represents the temperature dependence of the equilibrium binding between the analyte and receptor. We have applied this approach to several systems, including an aptamer system for arginine vasopressin (AVP), a hormonal polypeptide. Additionally, we are in the preliminary stages of developing a MEMS-based platform for label free investigation of thermally dependent kinetic properties (e.g., kon, koff and Kd) of analyte-receptor binding.
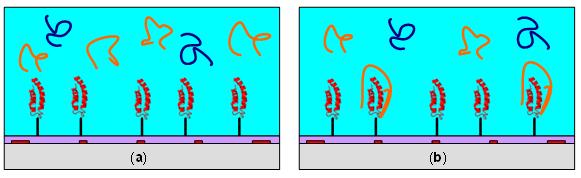 Principle of microfluidic characterization of temperature dependent biomolecular binding. (a) A sample of analyte and a non-binding reference is introduced to a receptor functionalized solid surface. (b) Depending on incubation temperature, a fraction of analytes bind to the receptor while unbound analytes and the reference remain in solution. This resulting solution is transferred for MALDI-MS to reveal a normalized mass spectral peak. 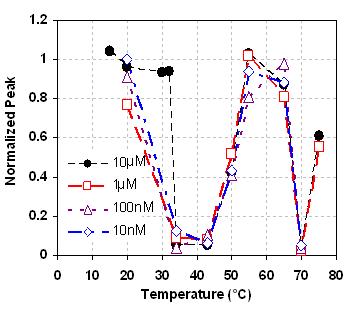 Temperature dependence of the equilibrium binding between AVP and an AVP-aptamer. We observe optimal binding (as indicated by very low normalized mass peaks) in two temperature zones, 34-45 °C and 70-75 °C. Dissociation of the spiegelmer from vasopressin (indicated by a high normalized peak) occurs in three temperature zones: 15-30 °C; 50-65 °C; and above 75 °C. Such complex binding profiles may be difficult to elucidate with conventional techniques.
|