| People | Research |
|
Microfluidic
Systems for aptamer-based affinity purification, enrichment, and label-free
detection The ability to isolate and detect trace amounts of analytes from complex samples is critically important to biological applications. We have been exploiting the integration of aptamer-functionalized solid surfaces within microfluidic devices for purification, enrichment and label-free detection of biomolecules. Our approach has clearly advantages in that affinity aptamer binding allows for specific capture and enrichment, and synthetic generability of aptamers makes our devices applicable to virtually limitless targets. This is in contrast to conventional solid-phase materials (e.g., reverse-phase or ion-exchange gels) that are non-specific and extract both analytes and impurities, and to other affinity receptors (e.g., antibodies and enzymes) that are derived from laboratory animals. Moreover, aptamer binding in general exhibits strong dependence on environmental stimuli. For example, the formation of secondary conformational structures by which aptamers specifically bind to targets is strongly temperature dependent. One of our unique contributions is to exploit such temperature dependence such that analytes can be captured and enriched at a predefined temperature, and subsequently released from the aptamers at a modestly different, yet also predefined temperature. This achieves isocratic elution (i.e., analytes are eluted in the same aqueous buffer in which analyte capture is performed) and eliminates the need for organic solvents, harsh acids or pH gradients, simplifying the experimental procedure and avoiding potentially compromising the integrity of analytes. Our microfluidic devices typically consist of a microchamber packed with aptamer-functionalized microbeads for analyte extraction and purification, a microheater and temperature sensor for thermally induced analyte release, and microchannels in conjunction with a surface tension-based valve for the control of sample flow. During operation, an analyte sample is extracted inside the aptamer microchamber via the sample inlet, while impurities are flushed away. Repeating this process enriches the analyte. Next, in the same aqueous buffer, the captured analyte is released from the aptamer surface by thermally induced aptamer-analyte dissociation, which also regenerates the aptamer surface for reuse if desired. The analyte is then isocratically eluted through a microflow gate onto a sample plate for label-free detection by matrix-assisted laser desorption/ionization mass spectrometry (MALDI-MS). Experimental results have demonstrated that various analytes can be specifically captured and enriched (e.g., by ~1000×), thermally released by a safely small temperature increase or decrease (e.g., < 10 °C) and detected distinctly by MALDI-MS.
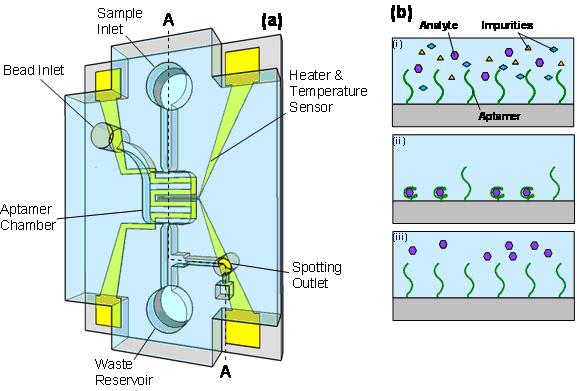 (a) A Microfluidic device for aptamer-based enrichment, thermally induced release and MALDI-MS detection of analytes. (b) Principle: i - an analyte solution is introduced to an aptamer functionalized surface; ii - the analyte interacts with the aptamer and is captured on the surface; iii - thermal activation of the surface disrupts the aptamer/analyte interaction allowing release. 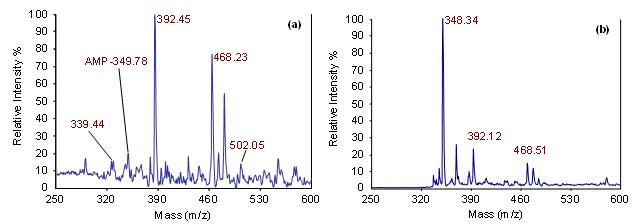 (a) MALDI-MS detection of a 10 nM concentration of adenosine monophosphate (AMP). The molecular ion peak of AMP is ~ 348 Da/z (MALDI matrix reference peaks are 339, 392, 468, and 502 Da/z). (b) Enrichment after 250 infusions of a 10 nM AMP sample revealing detection enhancement.
|